Leiden, The Netherlands, 7th June 2023 – Amarna Therapeutics, a privately-held biotechnology company developing transformative gene therapies in a range of rare and prevalent diseases, including Hemophilia B, today announces the appointment of Dr Henk Streefkerk as the Company’s new Chief Executive Officer and Medical Director. He succeeds Steen Klysner, who served as CEO for the past three years. Streefkerk already was Amarna’s Medical Director since mid-2022.
Dr Streefkerk has a distinguished career as CMO of several biotechs, including PIQUR Therapeutics, Cellprotera and Rivia, and brings over a decade of experience working in big pharma including Novartis, Actelion and Organon. As clinical pharmacologist/safety leader he led the global product safety team, overseeing safety analyses, regulatory submissions, and post-marketing safety surveillance for a heart failure drug. He also significantly contributed to the approval of an anti-cancer drug. He started his medical career in otorhinolaryngology and holds both MD and PhD qualifications.
“We are very pleased to introduce Henk Streefkerk as our new CEO. As a Physician Scientist he brings to Amarna a broad and extensive experience in clinical development, health authority negotiations, and other regulatory interactions. Henk will be instrumental in leading Amarna towards the first in human clinical trial,” said Thomas Eldered, Chairman of Amarna’s supervisory board.
“I’m thrilled to be joining Amarna as CEO. The company’s team has been working very hard to generate solid translational data. I look forward to work with them on this next phase of bringing Amarna’s personalized gene therapy product to hemophilia B patients and proving the potential of repeat dosing,” said Henk Streefkerk.
=== E N D S ===
About Amarna
Amarna Therapeutics is a privately held biotech company developing multiple-dose gene therapies to treat patients with genetic and idiopathic diseases, focusing first on hemophilia B. The company’s SVec gene delivery vector platform derived from the macaque polyomavirus SV40 is by design non-immunogenic and tolerogenic in humans inducing long-term transgene expression in patients with the possibility of repeated administration. The SVec platform could be applied in many different genetic and idiopathic diseases, including hemophilia A and B, cystic fibrosis, type 1 diabetes mellitus, multiple sclerosis and many others.
About Hemophilia B
The company’s lead project is on hemophilia B, an orphan recessive X-linked congenital bleeding disease, caused by mutations in the F9 gene encoding bleeding factor IX (FIX). This deficiency results in impaired hemostasis, prolonged bleeding and rebleeding. Based on the FIX levels in the blood patients are characterized as severe, moderate or mild hemophiliacs. Amarna’s SVec vector encoding human FIX moving towards its first-in-human clinical trial will restore the FIX levels in the blood of treated patients and through repeat dosing could meet many unmet patient needs.
More information on www.amarnatherapeutics.com
Follow us on LinkedIn
https://www.linkedin.com/company/amarna-therapeutics-b.v.
For further inquiries please contact:
Amarna Therapeutics
Henk Streefkerk, CEO
E-mail: info@amarnatherapeutics.com
LifeSpring Life Sciences Communication, Amsterdam
Léon Melens
Tel: +31 6 538 16 427
E-mail: lmelens@lifespring.nl
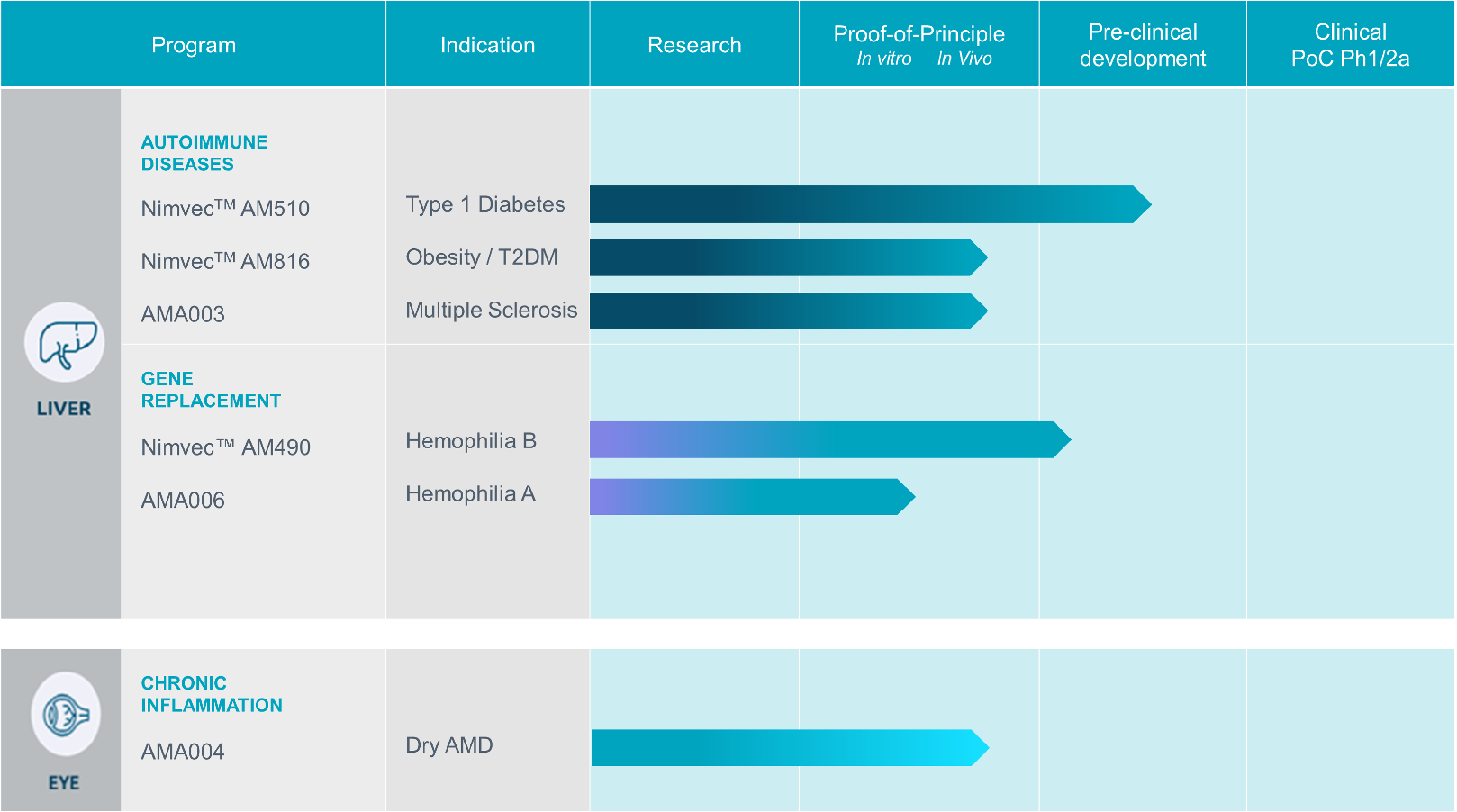
Classical Gene replacement therapy for monogenic indications, where Nimvec™ is used to deliver an active copy of a missing or dysfunctional gene. This is currently in development for a number of rare diseases, where Nimvec™ overcomes the limitations encountered by other vectors, mainly caused by immune reactions in humans.
Induction of tolerance towards specific self-proteins that are primary self-antigens in autoimmune diseases. Nimvec™-mediated delivery of primary self-antigens to a tolerizing setting, induces tolerance to the self-antigens to reestablish immune homeostasis and resolve the autoimmune condition.
Down-regulation of inflammation in chronic inflammatory diseases, by localized delivery of anti-inflammatory and regeneration promoting transgenes. Our current project is targeting a generic mechanism which will be applicable for a range of inflammatory indications.
Type 1 Diabetes
Nimvec™ AM510 is a tolerance inducing gene therapy for the treatment of Type 1 Diabetes. Diabetes is an autoimmune disease where self-reactive T lymphocytes selectively attack and destroy insulin-producing β cells lodged within the pancreas, leaving the patient unable to maintain glucose homeostasis. Proinsulin (PI) is considered to be the primary self-antigen involved in the autoimmune β cell destruction. To date, Type 1 Diabetes cannot be cured and the glucose homeostasis can be more or less maintained in patients by daily insulin injections. Although Diabetes is seen as a manageable disease nowadays, secondary complications of the current therapy are considerable and lead to significant morbidity and mortality. Using Nimvec™ AM510 we intend to restore the immune tolerance to proinsulin and potentially cure the patients.


Obesity / T2DM
Nimvec™ AM816 is a tolerance inducing gene therapy for the treatment of Obesity and T2DM. Obesity is a major risk factor for various health problems, including cardiovascular diseases, stroke, certain cancers, and respiratory issues
Multiple Sclerosis
AMA003 is a aim to develop a novel treatment of Multiple Sclerosis (MS). MS is an autoimmune disease where self-reactive T lymphocytes selectively attack and destroy oligodendrocytes leading to demyelination of axons in the brain and spinal cord that finally results in functional disability and premature death. Myelin components such as myelin oligodendrocyte glycoprotein (MOG) and myelin basic protein (MBP) are considered to represent the primary self-antigens involved in the chronic autoimmune destruction of oligodendrocytes. To date, MS cannot be cured. The current treatment options include the use of immune-suppressing antibodies to prevent the recurrence of acute attacks and to delay disease progression. However, long-term use of general immuno-suppressing drugs coincides with severe adverse side effects. Using AMA003 we intent to restore the immune tolerance to MOG and MBP and potentially cure the patients from the disease.


Hemophilia B
Nimvec™ AM490 is a gene replacement therapy product for the treatment of patients with HEMB. HEMA and HEMB are rare X-linked genetic disorders where mutations in the genes encoding FVIII and FIX, respectively, lead to impaired blood clotting. In hemophilia patients even a minor injury can result in severe and life-threatening blood loss. Currently, there is no cure for hemophilia and patients need regular infusions of the missing clotting factor. Gene replacement therapy using AAV vectors to deliver a functional copy of the gene encoding the clotting factor to the liver of patients has been shown to be a highly promising alternative to protein replacement therapy. using Nimvec™ AM490 we intend to restore the blood clotting process in HEMB patients using an Nimvec™ vector encoding human FIX.
Hemophilia A
AMA006 is a gene replacement therapy product for the treatment of patients with hemophilia A (HEMA). HEMA and hemophilia B (HEMB) are rare X-linked genetic disorders where mutations in the genes encoding blood clotting factor VIII (FVIII) and IX (FIX), respectively, lead to impaired blood clotting. In hemophilia patients even a minor injury can result in severe and life-threatening blood loss. Currently, there is no cure for hemophilia and patients need regular infusions of the missing clotting factor. Gene replacement therapy using AAV vectors to deliver a functional copy of the gene encoding the clotting factor to the liver of hemophilia patients has been shown to be a highly promising alternative to protein replacement therapy. In AMA006 we intend to restore the blood clotting process in HEMA patients using an Nimvec™ vector encoding human FVIII.
